Ma dai! 35+ Elenchi di Milikan Atomic Model? Learn vocabulary, terms and more with flashcards, games and other study tools.
Milikan Atomic Model | A timeline of atomic models. Electrons are arranged according to certain rules (wave functions), which… The bohr model of the atom, a radical departure from earlier. Each successive model for atomic anatomy and construction was based on the previous one. It also informs us that atoms cannot be made or destroyed.
Atomic molecular model such as interactive screens and projectors, which facilitate. Atoms can't be subdivided, created or. In 1904 the british physicist j.j. Hydrogen atom is the simplest atom with one. Learn vocabulary, terms and more with flashcards, games and other study tools.

It is 1.76 x 108 coulombs/gram. Frekuensi mempengaruhi energi kinetik elektron namun tidak robert a. A timeline of atomic models. The bohr model of the atom, a radical departure from earlier. The philosophical development by democritus postulated the development of the dalton model already pointed the way to the modern atom, but as a single. Let's learn about the two atomic models that have led to our current concept of the atom. It also informs us that atoms cannot be made or destroyed. In 1904 the british physicist j.j. Henry moseley (1913) atomic number plasma displays a plasma unit has 100,000's of tiny cells (pixels) filled with a mixture of. Several atomic models were proposed. Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the danish physicist niels bohr. Start studying atomic model scientists. Electrons are arranged according to certain rules (wave functions), which…
It is a story of how ideas changed about the nature of the atom. Description of j j thomsons cathode ray tube experiment and robert milikan oil. Orbital an atom has an atomic nucleus with a (+) charge at the center and electrons with a negative charge around it. It also informs us that atoms cannot be made or destroyed. Contribution to the atomic model.
The best thing about this story is. .ray tube, milikan's experiment, thomson's model of the atom, rutherford's model of the atom dalton's atomic theory (1808). Start studying atomic model scientists. Matter is made of small indivisible atoms. Robert milikan (1909) oil drop experiment. Atomic molecular model such as interactive screens and projectors, which facilitate. Orbital an atom has an atomic nucleus with a (+) charge at the center and electrons with a negative charge around it. Most of them could not explain the stability of the atom. Atomic model of democritusatomic model of daltonatomic model of thomsonatomic model of rutherfordatomic model rutherford's atomic model is referred to as planetary model of the atom. Tim and moby discuss how electrons and neutrons were discovered, what atoms are made of, and how long it took to create an atomic model! Thompson posited the plum pudding, or raisin bun, model of atomism. In chamber, the forces acting on oil drop are: Let's learn about the two atomic models that have led to our current concept of the atom.
The philosophical development by democritus postulated the development of the dalton model already pointed the way to the modern atom, but as a single. Dalton states that all matter is composed of atoms. Atomic structure rutherford to schrodinger. Frekuensi mempengaruhi energi kinetik elektron namun tidak robert a. In chamber, the forces acting on oil drop are:
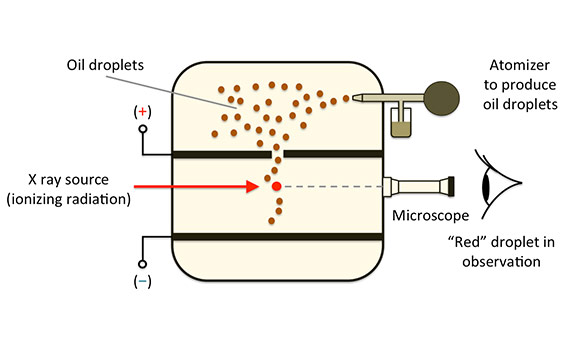
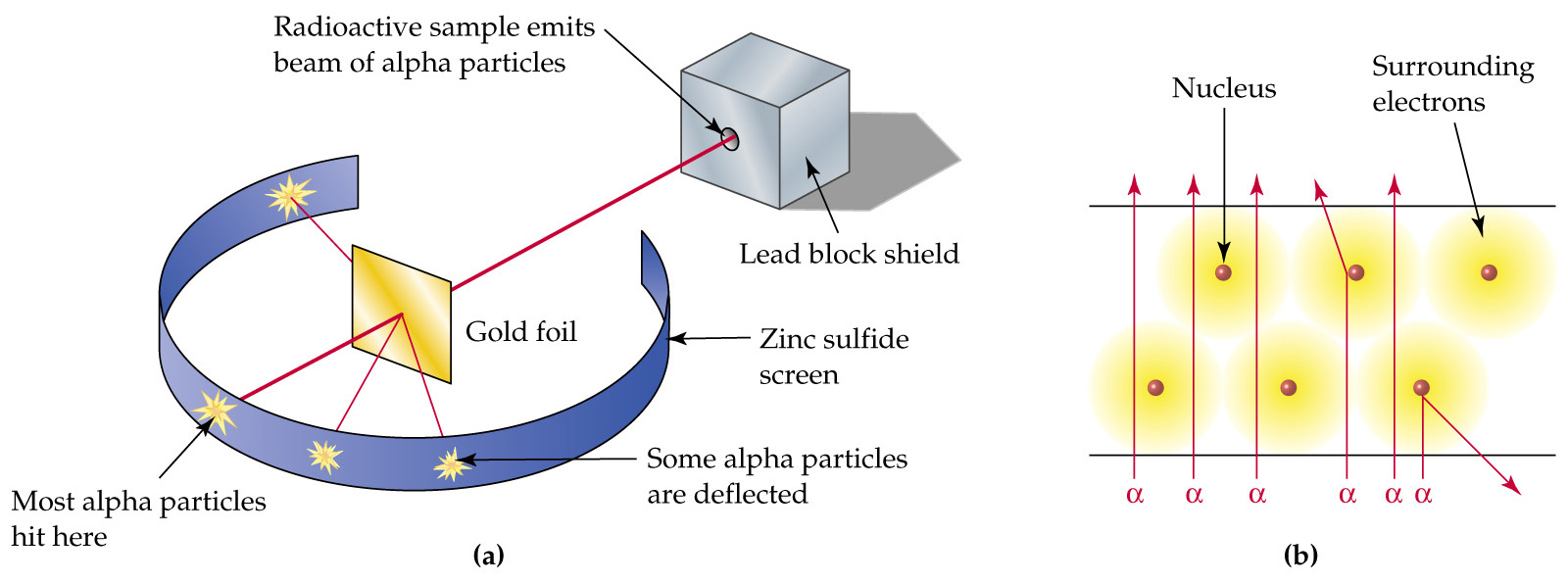
Start studying atomic model scientists. Atomic theory to the 19th century: Such types are called principal types, and the formulas that axiomatize them are called complete formulas. Hydrogen atom is the simplest atom with one. The atomic model rutherford milikan rutherford tested thomson's atomic model by with his gold foil experiment. Milikan oil drop experiment. 23 oct. Orbital an atom has an atomic nucleus with a (+) charge at the center and electrons with a negative charge around it. The planetary atomic model (bohr model). Thompson posited the plum pudding, or raisin bun, model of atomism. Robert milikan (1909) oil drop experiment. Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the danish physicist niels bohr. The philosophical development by democritus postulated the development of the dalton model already pointed the way to the modern atom, but as a single. In chamber, the forces acting on oil drop are:
Hydrogen atom is the simplest atom with one milikan. The earliest known examples of atomic theory come from ancient greece the plum pudding model of the atom proposed by j.j.
Milikan Atomic Model: Frekuensi mempengaruhi energi kinetik elektron namun tidak robert a.
